Submit adverse event report
Adverse events can be unexpected adverse events that occur during conduct of research or issues in regard to compliance. When an adverse event occurs, the chief investigator needs to submit an Adverse Event form.
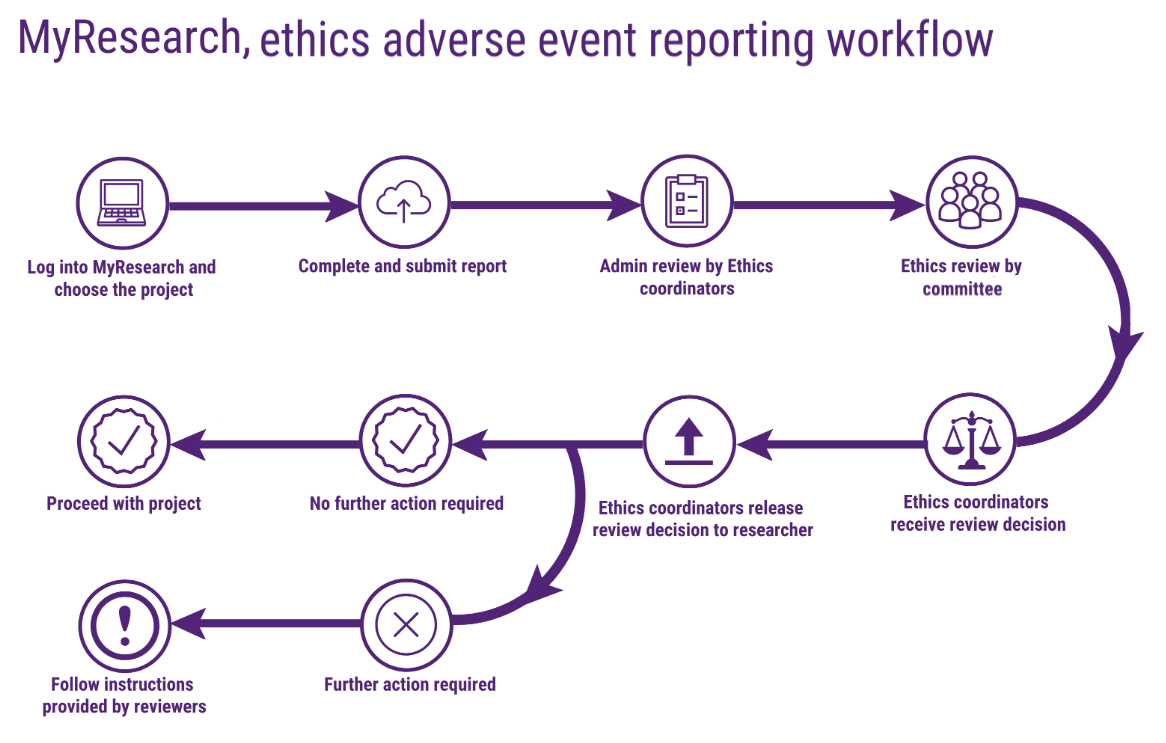
The diagram below shows the process steps for submitting an adverse event report.
Steps for submitting an adverse event report are as shown below:
1. Log in to the MyResearch website.
2. From the taskbar on your dashboard click on the project tab ![]() .
.
3. This will take you to the list of your projects. Choose on the title of the one for which you need to submit the report.
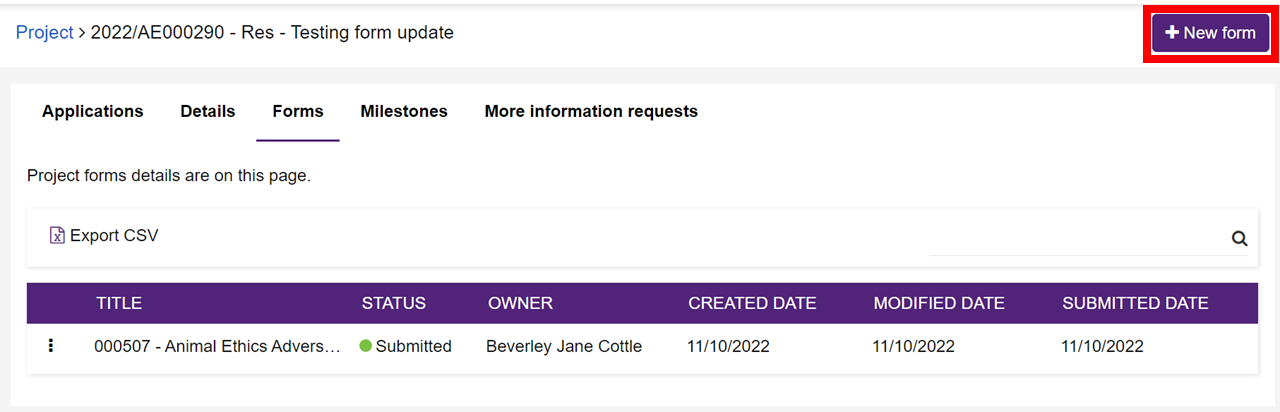
4. Click on the forms tab along the top of the page.
5. Click on new form in the top right hand corner.
6. Click on the form type you want to create. This will open up the adverse event form.
7. Complete the form, the form will save your progress as you go so you can complete it at a later date. All users with edit access to the form are able to start and contribute to Adverse Event report forms
8. When finished the Chief Investigator must click on submit.